Gifu University
University of Shizuoka
Discovery of the Enzyme
That Produces a Key Marker Molecule for the Diagnosis of X-linked Adrenoleukodystrophy
Key points
- We identified the enzyme involved in the production of the diagnostic marker for X-linked adrenoleukodystrophy, hexacosanoyl lysophosphatidylcholine (C26:0-LPC).
- Using molecular dynamics simulations, we elucidated the mechanism by which this enzyme functions at the atomic and molecular levels.
- Clarification of the biosynthetic mechanism of this diagnostic marker is expected to contribute to more accurate diagnosis of X-linked adrenoleukodystrophy and to a better understanding of the molecular basis underlying disease onset.
Overview
At present, hematopoietic stem cell transplantation※3 is the only treatment known to be effective. However, even with this therapy, it is difficult to restore brain function once it has been lost. Therefore, initiating treatment as early as possible—before neurological damage occurs—is critically important.
For this reason, screening programs for newborn infants (newborn screening※4) aimed at early detection of the disease have been implemented in the United States and Europe, and are being expanded in other regions. In these programs, a molecule called hexacosanoyl lysophosphatidylcholine (C26:0-LPC) is used as a diagnostic marker. However, the reason why this molecule accumulates in the bodies of patients with X-linked adrenoleukodystrophy has not been fully understood until now.
In this study, a research group led by Associate Professor Kotaro Hama of the Faculty of Pharmaceutical Sciences, Teikyo University, investigated how hexacosanoyl lysophosphatidylcholine (C26:0-LPC), a diagnostic marker for X-linked adrenoleukodystrophy, is produced within cells.
By focusing on hexacosanoyl phosphatidylcholine (C26:0-PC), a precursor molecule of C26:0-LPC, the researchers successfully identified an enzyme that plays a crucial role in the production of this diagnostic marker.
This research was conducted in collaboration with Yuko Fujiwara, Yoshio Kusakabe, Yasuhiro Hayashi, Shohei Azuma, Atsushi Yamashita and Kazuaki Yokoyama of Teikyo University; Specially Appointed Professor Nobuyuki Shimozawa of Gifu University; Associate Professor Shigeo Takashima of the Institute for Glyco-core Research; Professor Ryo Takita of the University of Shizuoka; graduate student Koko Imai; and Assistant Professor Masaru Kondo.
These findings are expected to contribute to improved diagnostic accuracy, including more precise prediction of disease progression. Furthermore, future studies are anticipated to clarify how C26:0-LPC itself is involved in the onset and progression of X-linked adrenoleukodystrophy.
Research Background
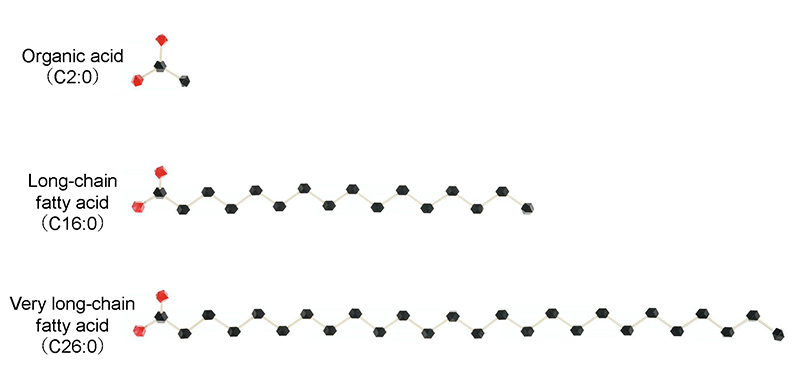
Figure 1. Fatty acids in the body
Fatty acids are basic components of fats and are made up of chains of carbon atoms. In the body, they are used as an energy source and also play important roles as building blocks of cell membranes and as starting materials for various biologically active substances. Fatty acids are grouped by the length of their carbon chains into short-, medium-, long-, and very long-chain fatty acids. The very long-chain fatty acid studied here has a chain of 26 carbon atoms.
X-linked adrenoleukodystrophy is characterized by progressive demyelination accompanied by inflammatory responses in the brain※5 (Figure 2), as well as by impaired adrenal function.
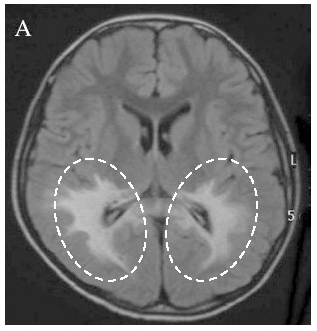
Figure 2. MRI image of a patient with X-linked adrenoleukodystrophy
In the brain of a patient with the cerebral form of X-linked adrenoleukodystrophy, areas of demyelination—where the protective covering of nerve fibers is damaged—can be seen (outlined by the dotted lines in the image). (Reproduced with permission from the Gifu University ALD & Peroxisomal Disease website)
For this reason, screening tests for newborn infants (newborn screening) have been implemented in the United States and Europe, and more recently in Japan. In these screening programs, hexacosanoyl lysophosphatidylcholine (C26:0-LPC) is used as a diagnostic marker (Figure 3). Through a combination of this screening and genetic testing of the transport protein responsible for very long-chain fatty acid metabolism, it has become possible to diagnose the disease at a presymptomatic or very early symptomatic stage.
However, discrepancies have been observed between genetic abnormalities in the transport protein and the severity of clinical manifestations of X-linked adrenoleukodystrophy, raising ethical concerns※6. To address this issue, it is essential to understand how the diagnostic marker C26:0-LPC is produced in the bodies of patients with X-linked adrenoleukodystrophy. Nevertheless, the molecular mechanism underlying its production has remained unclear until now.
Research Findings
In living organisms, enzymes known as lysophospholipid acyltransferases incorporate fatty acids into other lipid molecules (Figure 3). Based on this principle, the team proposed a working hypothesis that a lysophospholipid acyltransferase recognizes the very long-chain fatty acid C26:0 and synthesizes hexacosanoyl phosphatidylcholine (C26:0-PC). This C26:0-PC would then serve as the direct precursor for the generation of C26:0-LPC.
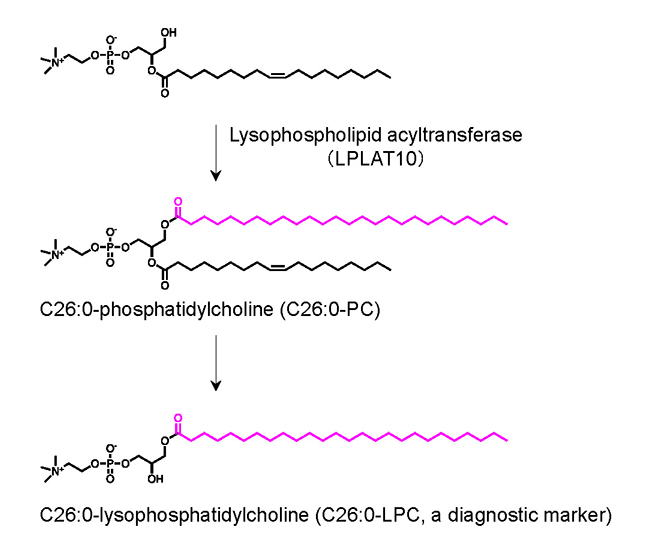
Figure 3. How C26:0-LPC is produced
This study found that C26:0-PC is first made in cells through a specific enzyme (LPLAT10). This substance is then further processed step by step inside the cell to produce C26:0-LPC, which is used as a marker to help diagnose the disease.
These findings support the concept that the cellular level of C26:0-LPC is determined by the abundance of its precursor, C26:0-PC.
Next, to gain a detailed understanding of how C26:0-PC is synthesized by LPLAT10, the research group conducted computer simulations based on publicly available three-dimensional protein structures. This analysis revealed a structural feature within LPLAT10 that stabilizes a portion of C26:0-PC.
Furthermore, using deuterium-labeled fatty acids※8 synthesized by an original method developed by the research group, the researchers successfully demonstrated the intracellular metabolic fate of excess C26:0 fatty acids.
Significance of the Research Findings
It is known that very long-chain fatty acids accumulate in patients with X-linked adrenoleukodystrophy; however, the mechanisms by which these fatty acids contribute to disease pathology remain incompletely understood. In this study, the researchers identified an enzyme that specifically recognizes very long-chain fatty acids and clarified part of their metabolic pathway. These findings are expected to further advance our understanding of the toxicity of very long-chain fatty acids and the molecular mechanisms underlying disease development in X-linked adrenoleukodystrophy.
Funding sources
The results of this study were published in the Journal of Lipid Research on December 30, 2025.
Title:Phosphatidylcholine with C26:0-moiety, a precursor of a diagnostic marker for X-ALD, is synthesized by LPLAT10/LPEAT2
Authors:Kotaro Hama*, Yuko Fujiwara, Koko Imai, Yoshio Kusakabe, Yasuhiro Hayashi, Shigeo Takashima, Shohei Azuma, Masaru Kondo, Atsushi Yamashita, Ryo Takita, Nobuyuki Shimozawa, and Kazuaki Yokoyama (* Corresponding author)
DOI:https://doi.org/10.1016/j.jlr.2025.100973
URL:https://www.jlr.org/
Glossary
A genetic metabolic disorder in which very long-chain fatty acids accumulate in the body, causing inflammation and demyelination in the brain and progressive neurological symptoms. Because severe cases can be life-threatening, early diagnosis and timely intervention are essential.
※2 Adrenal gland:
A small organ above the kidneys that produces hormones essential for stress response, metabolism, and blood pressure regulation.
※3 Hematopoietic stem cell transplantation:
A treatment that transplants blood-forming stem cells to restore blood and immune function and help slow disease progression.
※4 Newborn screening:
A test using a small blood sample from newborns to detect congenital disorders early and enable timely treatment before symptoms appear. Newborn screening for X-linked adrenoleukodystrophy was first introduced in the United States.
※5 Demyelination:
A condition in which the protective myelin sheath around nerve fibers is damaged, disrupting nerve signal transmission.
※6 Ethical considerations:
Newborn screening for X-linked adrenoleukodystrophy does not always allow accurate prediction of disease onset or severity, and some infants who may never develop symptoms can be identified. In addition, genetic testing may reveal variants of uncertain significance or unexpected findings, highlighting the need for appropriate information provision and genetic counseling.
※7 Genome editing technology:
A technique that precisely modifies specific DNA sequences to study gene function and disease mechanisms. This study used the CRISPR-Cas system.
※8 Deuterium-labeled fatty acids:
Fatty acids in which some hydrogen atoms are replaced with deuterium, a stable isotope, allowing researchers to track fatty acid metabolism in cells. Using an in-house method, the researchers successfully synthesized a deuterium-labeled very long-chain fatty acid (C26:0).
Contact Information
Faculty of Pharmaceutical Sciences, Teikyo University
Advanced Comprehensive Research Organization, Teikyo University
Kotaro Hama, Associate Professor
Tel: +81-3-3964-8197
Fax: +81-3-3964-8198
E-mail: khama(Insert @ here)pharm.teikyo-u.ac.jp
Kazuaki Yokoyama, Professor
Faculty of Pharmaceutical Sciences, Teikyo University
E-mail: yokoyama(Insert @ here)pharm.teikyo-u.ac.jp
For Media Inquiries:
Public Relations Office, Teikyo University
Tel: +81-3-3964-4162
E-mail: kouhou(Insert @ here)teikyo-u.ac.jp
Public Relations and Planning Office, University of Shizuoka
Tel: +81-54-264-5130
E-mail: koho(Insert @ here)u-shizuoka-ken.ac.jp
(2/4/2026)


